Research
Development of novel chiral catalysts and their application to highly enantioselective reactions:
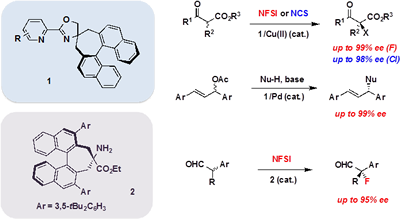
We have been studying the development of novel chiral transition-metal catalysts and organocatalysts. For example, we synthesized a new class of chiral oxazoline ligands 1 with a spiro structure and successfully applied them to various metal-catalyzed asymmetric transformations giving high enantioselectivity, such as Cu(II)-catalyzed enantioselective halogenation and Pd(0)-catalyzed CーC bond forming reactions. Recently, we also developed a new chiral primary amine catalyst 2 having an axial chiral binaphthyl backbone, which catalyzed the asymmetric fluorination of a-branched aldehydes with high enantioselectivity. These reactions could be utilized to the asymmetric synthesis of biologically active compounds.
Asymmetric synthesis of chiral halogenated compounds and their derivatization via the stereospecific carbon-halogen bond cleavage
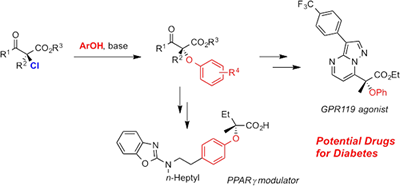
We achieved highly enantioselective halogenation reactions with the above-mentioned chiral catalysts, which have had difficulty in achieving high enantioselectivity so far. We also achieved the derivatization of the resulting halogenated compounds via stereospecific carbon-halogen bond cleavage. In particular, the SN2 substitution of α-chloro-β-keto esters proceeded smoothly despite the fact that the reaction occurred at a tertiary carbon center. Using this method, we demonstrated the synthesis of a potential treatment for type 2 diabetes.
Selected Papers:
- H. Mizutani, R. Kawanishi, K. Shibatomi*, Chem. Commun. 2021, 57, 6676-6679.
- R. Kawanishi, K. Nakada, K. Shibatomi*, Beilstein J. Org. Chem. 2021, 17, 229-233.
- M. K. Kam, A. Sugiyama, R. Kawanishi, K. Shibatomi*, Molecules. 2020, 25, 3902.
- R. Kawanishi, S. Hattori, S. Iwasa, K. Shibatomi*, Molecules. 2019, 24, 2773.
- R. Kawanishi, L. Phongphane, S. Iwasa, K. Shibatomi*, Chem. Eur. J. 2019, 25, 7453-7456.
- A. Naruse, K. Kitahara, S. Iwasa, K. Shibatomi*, Asian J. Org. Chem. 2019, 8, 691-693.
- M. Katada, K. Kitahara, S. Iwasa, K. Shibatomi*, Synlett. 2018, 29, 2408–2411.
- K. Shibatomi*, K. Kitahara, N. Sasaki, Y. Kawasaki, I. Fujisawa, S. Iwasa, Nature Commun. 2017, 8, 15600.
- K. Shibatomi*, K. Kitahara, T. Okimi, Y. Abe, S. Iwasa, Chem. Sci. 2016, 7, 1388-1392.
- K. Shibatomi*, M. Kotozaki, N. Sasaki, I. Fujisawa, S. Iwasa, Chem. Eur. J. 2015, 21, 14095-14098.
- K. Shibatomi*, Y. Kawasaki, S. Iwasa, J. Fluorine Chem. 2015, 179, 77-82.
- K. Shibatomi*, T. Okimi, Y. Abe, A. Narayama, N. Nakamura, S. Iwasa, Beilstein J. Org. Chem. 2014, 10, 323-331.
- K. Shibatomi*, Y. Soga, T. Muto, S. Iwasa, Synlett 2013, 24, 375-378.
- K. Shibatomi*, Y. Soga, A. Narayama, I. Fujisawa, S. Iwasa, J. Am. Chem. Soc. 2012, 134, 9836-9839.
- K. Shibatomi*, A. Narayama, Y. Abe, S. Iwasa, Chem. Commun. 2012, 48, 7380-7382.
- K. Shibatomi*, F. Kobayashi, A. Narayama, I. Fujisawa, S. Iwasa, Chem. Commun. 2012, 48, 413-415.
- K. Shibatomi*, A. Narayama, Y. Soga, T. Muto, S. Iwasa, Org. Lett. 2011, 13, 2944-2947.
- K. Shibatomi*, K. Futatsugi, F. Kobayashi, S. Iwasa, H. Yamamoto*, J. Am. Chem. Soc. 2010, 132, 5625-5627.
- K. Shibatomi*, T. Muto, Y. Sumikawa, A. Narayama, S. Iwasa, Synlett 2009, 241-244.
- K. Shibatomi*, Y. Tsuzuki, S. Iwasa*, Chem. Lett. 2008, 37, 1098-1099.
- K. Shibatomi*, H. Yamamoto*, Angew. Chem. Int. Ed. 2008, 47, 5796-5798.
- K. Shibatomi*, Y. Zhang, H. Yamamoto*, Chem. Asian J. 2008, 3, 1581-1584.
- K. Shibatomi, Y. Tsuzuki, S.-I. Nakata, Y. Sumikawa, S. Iwasa*, Synlett. 2007, 551-554.
